About FDA Form 1572
FDA Form 1572 is a mandated form that provide sponsors with relevant information about clinical trials and to assure the U.S. Food and Drug Administration (FDA) of compliance with all regulations. The process of completing the form can be burdensome, time-consuming, and frustrating.
The Challenge of FDA Form 1572
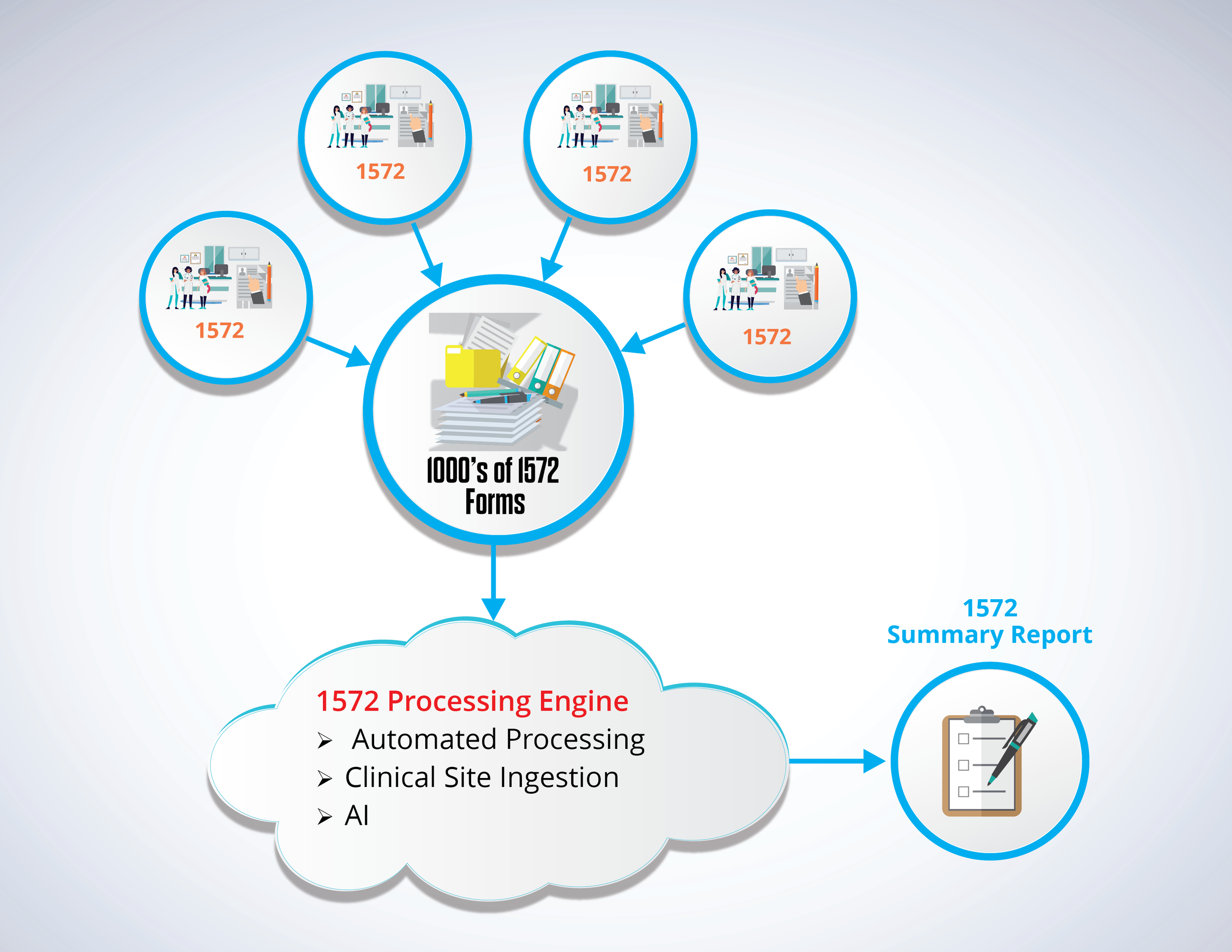
Manually combing through and combining information from hundreds of FDA 1572 forms generated across multiple sites and over a significant span of time into a summary report, requires clinical trial leads to spend hundreds of man hours on this single task. This standardized form has numerous boxes for administrative personnel to enter information. However that information could be entered in numerous ways and it usually takes a human to correlate that information over multiple years of a clinical study.
Streamlined 1572 Solution
Court Square Group’ solution uses optical character recognition (OCR), artificial intelligence (AI), and customized coding to comb through all data listed on each FDA 1572 form and pulls out all relevant data needed for the clinical summary report (CSR). The software is able to reconcile misspellings, understand different syntax for identical names and there is no preprocessing or manual effort required by the end user.
This streamlined solution iterates over all clinical sites until it has created a comprehensive CSR. This is then returned to the clinical trial lead for a final review. The built-in artificial intelligence (AI) matches all variations of names that may be identical and thus need to be merged for the final report. During this review process, the clinical trial lead can easily confirm or reject those matches with the click of a button. The clinical trial lead will then simply audit the report to ensure there is no missing information and reconcile any discrepancies in each site’s section of the report.
Court Square Group’s 1572 solution is a secure and cost-effective way to ensure this important information is handled carefully throughout the review compilation process. Regardless of whether or not an organization utilizes a clinical trial management system (CTMS), this solution will significantly cut down the manpower, errors, and time it has previously taken for clinical trial leads to create a comprehensive summary report for submission.
Let’s start a conversation. (413) 746-0054